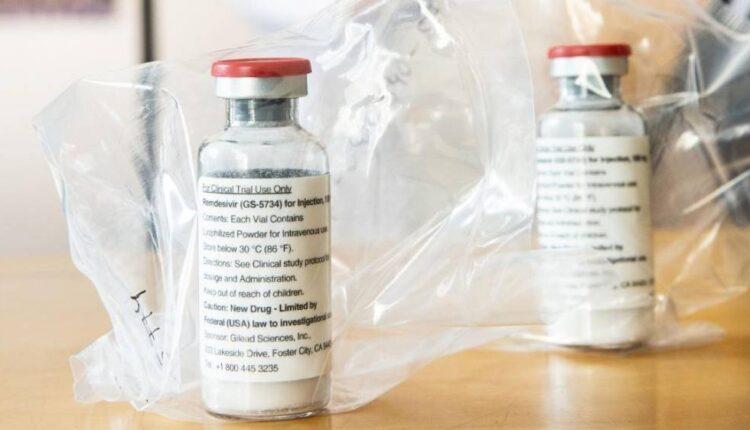
(CNN)A five-day course of the antiviral drug remdesivir sped recovery in moderately ill patients with pneumonia from Covid-19, drugmaker Gilead Sciences announced in a statement Monday.
It’s more evidence that the drug can help patients, however outside experts are not calling this a “game changer” quite yet.Coronavirus patients who were hospitalized, but not sick enough to need oxygen from a ventilator, were more likely to recover after a five-day course of remdesivir than those given the current standard of care alone, Gilead said.
JUST WATCHED
Hospitals frustrated by remdesivir supply
ReplayMore Videos …MUST WATCH
Hospitals frustrated by remdesivir supply 02:30The results from the Phase III clinical trial on the drug have not yet been published in a peer-reviewed medical journal. Gilead said it plans to submit the full data for publication in the coming weeks.”With the latest data announced today, we now have three randomized, controlled clinical trials demonstrating that remdesivir improved clinical outcomes by several different measures,” Dr. Merdad Parsey, chief medical officer for Gilead Sciences, said in a company statement. Read MoreGilead originally studied remdesivir as a potential treatment for Ebola, but lab experiments suggested it might be active against coronavirus.


JUST WATCHED
Dr. Gupta: right now remdesivir is the only treatment out there, as FDA gives authority for emergency use of the drug
ReplayMore Videos …MUST WATCH
Dr. Gupta: right now remdesivir is the only treatment out there, as FDA gives authority for emergency use of the drug 07:08The US Food and Drug Administration has not approved any treatment for Covid-19 — but in early May, the FDA issued an emergency use authorization for remdesivir for use as a treatment for the sickest hospitalized Covid-19 patients. Remdesivir is approved to treat Covid-19 in Japan, but is considered an investigational treatment for Covid-19 elsewhere in the world, Gilead noted.”With the additional data we have in hand today, we will continue to pursue research opportunities to evaluate patient outcomes and potentially benefit more patients with remdesivir,” Parsey said.”These include extending treatment earlier in the course of disease, combination studies with other therapies for the most critically ill patients, pediatric studies and the development of alternate formulations,” Parsey said. “We will continue to share emerging data with regulatory authorities as we work together to help address the needs of patients around the world.”
Some improvement with five-day course of treatment, early data suggest
For the new study, led and funded by Gilead, researchers randomly assigned 600 patients to receive either the current standard of care for Covid-19; a five-day course of remdesivir in addition to the current standard of care; or 10 days of the drug plus care. The recovery of each patient was tracked and examined using a scale.Moderately ill Covid-19 patients who received a five-day course of remdesivir were more likely to have clinical improvement after 11 days compared to those who received traditional standard of care alone, Gilead said. However, while patients who received a 10-day course of the drug improved, the results were not statistically significant, suggesting just five days of treatment may be enough to help patients recover. The full data still needs to be released.Gilead said that within 11 days, 134 of the 191 patients given a five-course of remdesivir showed at least two points of improvement on the tracking scale, and no one died. And 126 of the 193 patients who were given a 10-day course showed at least two points of improvement on the scale, but two died. Among the 200 patients who received only the standard of care, 121 showed at least two points of improvement and four died.The most common side effects of remdesivir in the study were nausea, diarrhea and headaches.”If we can intervene earlier in the disease process with a five day treatment course, we can significantly improve clinical outcomes for these patients,” Dr. Francisco Marty, an infectious disease physician at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School, who worked on the trial, said in Gilead’s press release.When asked on Twitter on Monday why the data for a 10-day course looked no better than the standard of care, Marty responded that it “could be a toxicity that plays out with the longer dosing” or “a fluke of open-label design & physician behavior, where clinicians go longer for sicker patients.”
‘These improvements are not dramatic’ and ‘not a game changer’
The improvements were not dramatic, Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine in the United Kingdom, said in a written statement released by the UK-based Science Media Centre on Monday.”The data show that there is only a small difference between the groups in improvement,” Evans said.”These improvements are not dramatic – they are not a ‘game changer’ in the terrible jargon, but at least there is some genuine evidence of improvement. For the patients it is good news that few died, but the evidence therefore that remdesivir improves mortality in these patients is uncertain and limited,” Evans added.”Remdesivir is one of the only drugs to show some promise, but the benefits are not large and we need to have more transparent data before we can form a good judgment.”Last month, the New England Journal of Medicine published results from a different remdesivir study evaluating the drug in patients who were hospitalized with severe Covid-19, including those on mechanical ventilation. Get CNN Health's weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
That data showed the drug shortened the course of illness from an average of 15 days to about 11. The researchers, led by a team at the National Institute of Allergy and Infectious Diseases, added that “given high mortality despite the use of remdesivir, it is clear that treatment with an antiviral drug alone is not likely to be sufficient.”
Source: edition.cnn.com